Abstract
Introduction.
Tumor infiltrating T cells (TILs) are capable of recognising tumor-specific mutations presented as peptides on MHC class I. Overexpression of immune checkpoint ligands on cancer cells, and the corresponding expression of the receptors on T cells, appears to be an important mechanism of immune evasion, exploited with success in immune checkpoint therapy. However, when the cancer cell is itself a T cell, the microenvironment interaction is likely to be more complex. To investigate this further we utilized the clonal nature of mycosis fungoides (MF), a primarily CD4 T cell cancer, by targeting the TCR receptor, differentiating between TIL and tumor in the microenvironment.
Methods.
43 patients with mycosis fungoides (MF) were prospectively consented to the study, and a skin biopsy and blood sample were obtained. The skin biopsy was macerated and digested with collagenase D, and a TCR V-beta clonogram was used to identify the tumor population. Flow cytometry was used to isolate five cell populations: peripheral blood CD4 and CD8, TIL CD4 and CD8, and tumor cells. The expression of a panel of immune checkpoint receptors (PD-1, TIM-3, LAG-3, Fas & TIGIT) and ligands (PD-L1, PD-L2, Galectin 9, Fas ligand) were measured using conjugated antibodies.
Results.
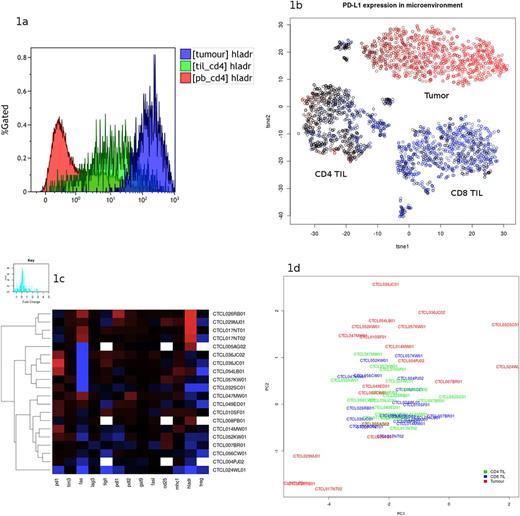
46 biopsies from MF patients with stage IA(n=6), IB(n=18), IIA(n=1), IIB(N=14), IIA(n=1) & IVA(n=3) were analysed with paired blood. The technique was effective in distinguishing tumour from TIL in 20 cases, isolating a population with CD7 downregulation, high forward and side scatter (indicative of larger and more complex cells) and a unique immune checkpoint profile (figure 1a & 1b). Tumour infiltrating lymphocytes of both CD4 and CD8 phenotype demonstrated significant overexpression of immune checkpoint receptors PD-1 (p < 0.0001), TIGIT (p < 0.0001) & fas (p < 0.0001), with an increased population of T regulatory cells in the CD4 cells (p < 0.001) and CD8 expressing high TIM-3 (p < 0.0001) compared to peripheral blood. While the TIL population was similar across the demographic, the tumour population demonstrated a significantly varied phenotype, with up and down-regulation of immune checkpoints and HLA-DR (figure 1c). Principal component analysis (figure 1d) revealed three tumour subtypes:
1. High PD-L1, Fas, HLA-DR (n=4)
2. High PD-1. Low PD-L1 (n=12)
3. Low PD-1, PD-L1, Fas (n=3)
These subtypes did not relate to disease type, stage, presence of large cell transformation or folliculotropic disease, though those with advanced disease had tumor phenotypes which differed more from the TILs. Tumor cells not only expressed MHC class I and HLA-DR, but upregulated these compared to peripheral blood (p < 0.001).
Discussion.
The TIL phenotype in MF is surprisingly homogeneous across the demographic, demonstrating upregulation of immune checkpoint receptors and an exhaustion phenotype seen in other cancers. However, the tumor cells did not show consistent upregulation of immune checkpoint ligands, instead their phenotype across patients was heterogeneous with both up and down-regulation. Expression of immune checkpoint ligands by the tumor cells may have negative growth consequences for both tumor cells as well as the immune system, particular since tumor T cells often express immune checkpoint receptors. We did not see concurrent high expression of PD-L1 and PD-1 on tumor cells, which would be expected to be detrimental to tumor survival. Immune checkpoint ligand over-expression is unlikely to be the main method of immune evasion in mycosis fungoides, and as a result, immune checkpoint inhibition may have unexpected responses in this disease.
Legends.
Figure 1a. TCR V-beta antibody used to separate three CD4 populations (red: peripheral blood CD4, green: CD4 TIL, blue: tumor) and compare HLA-DR expression.
Figure 1b. tSNE clustering of cells in the tumour microenvironment, demonstrating clear separation of three populations - tumor, CD4 TIL and CD8 TIL. Coloring by expression of PD-L1 (red: high, blue: low).
Figure 1c. Heatmap of immune checkpoint markers (red: high, blue: low) in the tumor cell population only. This demonstrates the heterogeneity, particularly in PD-1, Fas, PD-L1 and HLA-DR expression.
Figure 1d. Principal component analysis demonstrating the observation that TIL expression of immune checkpoints are similar between patients (green and blue), whereas tumor expression of these markers (red) can vary markedly and cluster.
Scarisbrick: NHS: Employment; Millennium Pharmaceuticals, Inc.: Consultancy, Honoraria; Mallinckrodt: Consultancy, Honoraria; Innate Pharma: Consultancy; Actelion: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal